The Ultimate Guide to Choosing the Right 3D Printing Service
The success of medical device manufacturing depends on efficiency, regulatory compliance, and precision. From initial concept to final production, manufacturers must carefully navigate every step to reduce risks, meet market demands, and maintain product quality.
Refining each stage of development helps companies optimize their operations. Working with the right partners can improve time-to-market.
Early Planning for Manufacturing Success
One of the most effective ways to optimize production is to involve manufacturing experts early in the design process. By doing so, potential challenges related to cost, production techniques, and scalability can be identified and addressed before they become major setbacks.
Design engineers must collaborate closely with manufacturing teams to assess material selection, tooling requirements, and production feasibility.
Proper planning also includes analyzing market demands and setting realistic production cost targets. A well-defined roadmap helps in making informed decisions that prevent unnecessary modifications and delays in later stages. When cost expectations align with manufacturing capabilities, efficiency improves, and the risk of redesigns decreases.
Streamlining Product Design for Efficiency
A streamlined product design not only enhances functionality but also makes manufacturing more efficient. Overly complex designs can lead to difficulties in production, higher defect rates, and increased costs.
Simplifying components while maintaining performance makes sure that devices can be manufactured consistently. This approach also enhances reliability.
Working with experienced engineers to refine design elements can result in a more manufacturable product. Features such as snap-fit connections instead of screws, fewer moving parts, and standardized materials all contribute to smoother production. A strong focus on design optimization helps prevent bottlenecks and enhances scalability for high-volume manufacturing.
Choosing The Right Materials and Components
Material selection plays a crucial role in medical device production. Factors such as biocompatibility, sterilization compatibility, and mechanical properties determine the durability and safety of the final product. Selecting readily available and cost-effective materials minimizes supply chain disruptions and production delays.
In addition to materials, sourcing high-quality components from trusted suppliers is essential. Many medical devices require custom components, including precision springs, wires, and tubing. Partnering with manufacturers that specialize in these parts make sure of consistency and compliance with regulatory standards.
Testing materials for durability and compliance early in the process avoids costly rework later. Manufacturers should conduct rigorous testing for chemical resistance, heat tolerance, and mechanical integrity to confirm that the selected materials meet industry and regulatory standards.
Additionally, working with suppliers that maintain consistent quality control makes sure a steady supply of reliable materials for production.
Implementing Advanced Manufacturing Techniques
Adopting advanced manufacturing techniques can significantly enhance efficiency and product quality. Technologies such as automation, 3D printing, and computer-aided manufacturing reduce manual errors and improve precision. Automation streamlines assembly processes and cuts down on labor costs while increasing output consistency.
Additionally, rapid prototyping allows for quicker iteration and testing. It shortens development cycles and accelerates product launches. Investing in these advanced techniques can lead to cost savings and improved market competitiveness in the long run.
Manufacturers that integrate Industry 4.0 technologies—such as the Internet of Things (IoT), artificial intelligence, and predictive maintenance—can further optimize their production processes. Real-time data monitoring helps detect inefficiencies early.
Smart automation enables flexible production lines that can quickly adapt to design modifications. This brings higher efficiency and responsiveness to market demands.
Guaranteeing Regulatory Compliance and Quality Control
Regulatory requirements in medical device production are stringent. This makes compliance a top priority. The FDA, along with other global regulatory bodies, enforces strict guidelines to make sure safety and efficacy. A proactive approach to compliance includes thorough documentation, testing protocols, and risk management strategies.
Quality control measures must be embedded throughout the production process. Regular audits, inspections, and adherence to Good Manufacturing Practices (GMP) reduce the chances of defects and recalls. Companies can maintain compliance and build trust with healthcare professionals and patients by implementing rigorous quality management systems.
Standard operating procedures (SOPs) should be developed for every step of the production process to maintain consistency and make sure of regulatory alignment.
Additionally, utilizing digital record-keeping systems allows for efficient tracking of compliance documentation. This will help simplify audits and reduce the risk of errors in record management.
Partnering With an Experienced Manufacturer
Selecting the right manufacturing partner is crucial for optimizing the production process. An experienced medical device manufacturer offers expertise in design refinement, material selection, and process validation. Collaborating with a partner who understands industry regulations makes sure that all compliance requirements are met seamlessly.
A reliable manufacturing partner offers transparency, scalability, and support throughout the entire production cycle. Be it for prototyping, small-batch production, or large-scale manufacturing, having a trusted collaborator enhances efficiency and accelerates time-to-market.
Manufacturers should assess potential partners based on their track record, certifications, and ability to meet industry standards. A strong partnership fosters innovation. Experienced manufacturers often bring valuable insights into process improvements and emerging technologies.
Continuous Improvement for Long-Term Success
Optimization is an ongoing process that requires continuous monitoring and refinement. Regular assessments of production workflows help identify inefficiencies and areas for improvement. Implementing lean manufacturing principles and investing in employee training fosters innovation and operational excellence.
Feedback loops between design teams, production units, and end-users allow for iterative improvements that enhance product performance and manufacturability. Companies that prioritize ongoing optimization stay ahead of industry changes. This helps them maintain a competitive edge in the medical device sector.
Tracking key performance indicators (KPIs), such as defect rates, production times, and equipment downtime, offers measurable insights into operational efficiency. Identifying trends and addressing inefficiencies through data-driven decision-making enhances long-term productivity and cost-effectiveness.
Additionally, investing in research and development (R&D) allows manufacturers to stay ahead of technological advancements and regulatory updates.
Streamlining Medical Device Manufacturing for Faster, High-Quality Production
Optimizing the medical device manufacturing process involves strategic planning, design refinement, material selection, regulatory compliance, and strong partnerships. With advanced manufacturing techniques and continuously improving workflows, companies can enhance efficiency, reduce costs, and deliver high-quality products to market faster.
RMA Engineering, LLC specializes in supporting medical device companies throughout the entire production journey. From early-stage development to full-scale manufacturing, our expertise makes sure of streamlined processes, quality assurance, and compliance with industry standards.
Reach out to us today to discuss how we can help optimize your manufacturing process.
Recent Posts

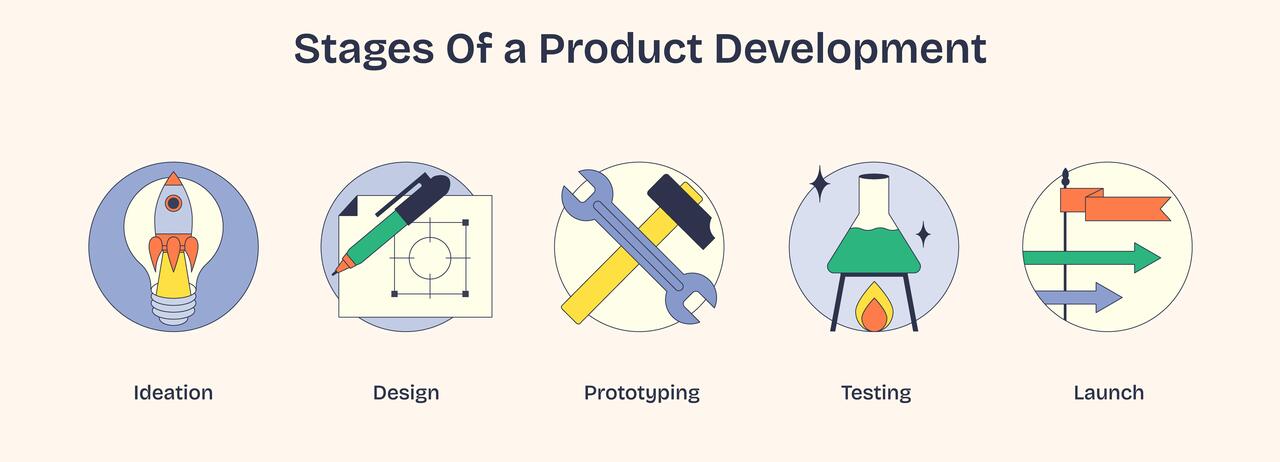
The Key Stages Of Product Development: A Complete Guide

How To Design A Product That Stands Out In The Market
Contact Us
For custom quotes please fill out the form below. Or email us at info@rmaengineering.tech.

